Part 5: MECHANISMS
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
General common errors:
(1) incorrect formal charges (2) backwards arrows (3) not showing the arrows for all the bonding changes (4) misuse of resonance / equilibrium arrows (5) vague arrows e.g. not starting on a bond or lone pair.
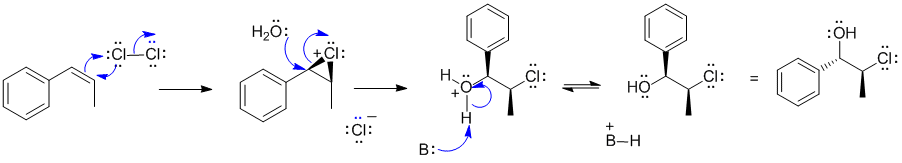
A1 Halohydration of an alkene:

Here B: could be water or the chloride ion.
Common errors:
A2 Cyclopropantion of an alkene with a carbene:
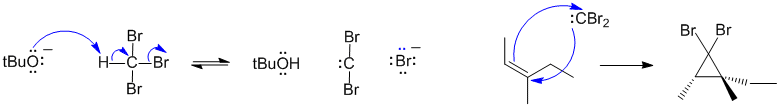
B1
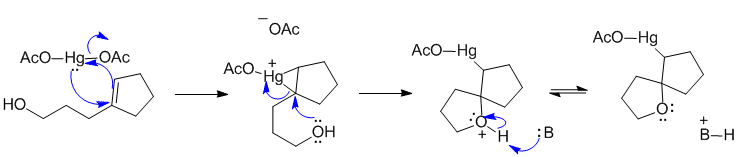
Here B: could be the acetate ion.
B2
Alkene protonates and then there is a 1,2-alkyl shift to create a more stable tertiary carbocation, then loss of H+ to give the most highly substituted alkene which is more stable:
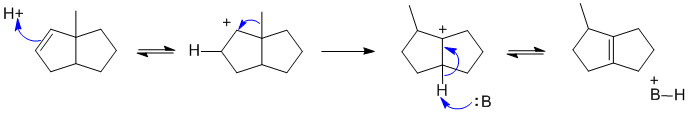
Here B: could be the alkene or the conjugate base of the acid catalyst.