Part
7: LABORATORY
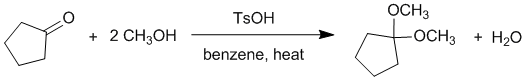
Experimental yields.... First you need to balance the reaction equation and then work out the moles of each reagent used to determine the limiting reagent:
Note that TsOH (tosic acid) is just a catalyst and benzene is the solvent and therefore can be ignored in terms of the yield calculation.
| |
|
| MF |
C5H8O |
CH4O |
|
C7H14O2 |
|
| MW (g/mol) |
84.12 |
32.04 |
|
130.18 |
|
| amount (g) |
8.412 |
7 x 0.79 = 5.53 |
|
10 |
|
| mmoles |
100 |
172 |
|
76.8 |
|
| mmoles/ coefficient |
100 |
172 / 2 = 86 |
|
76.8 |
|
Therefore, in the last row of the table where the stoichiometric coefficient from the balanced equation is divided into the number of moles, we can see that the limiting reagent in the alcohol. This means that the maximum amount of product that can be formed is 86 mmol (based on the reaction stoichiometry). Hence the % yield, based on obtained / max. possible = 76.8 / 86 = 89 %.
Common general errors: (1) not balancing the reaction equation, (2) not determining the molecular formulae and / or molecular weight correctly (3) not determining the limiting reagent correctly and (4) not knowing how the calculate an experimental yield. Note that it doesn't matter whether you work with grams or moles of product the answer should be the same when done correctly.