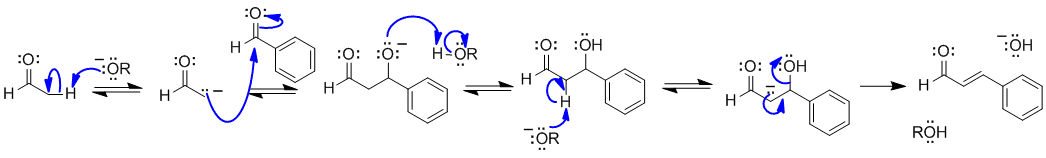
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
A1 An aldol condensation

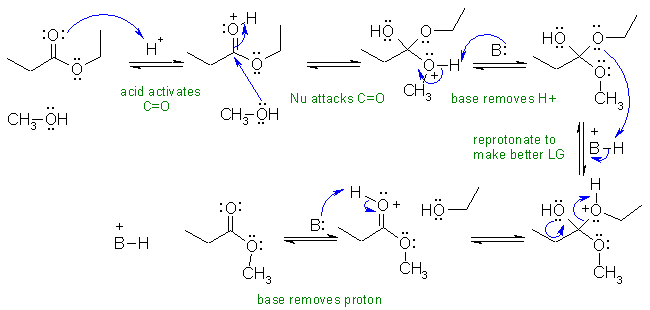
A2 Acid catalysed transesterification
B1
Bromine initially adds to the alkene in the normal manner to give the cyclic brominium ion. But then the very nucleophilic S atom that is also in close proximity acts as the nucleophile to open the bromonium ion. This results in a similar type of the structure that the nucleophilic bromide ion can react with to give the required regiochemistry.
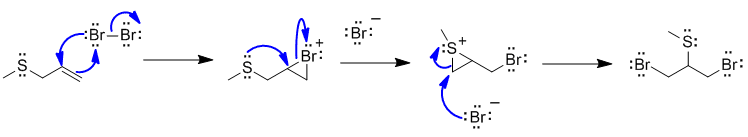
Common errors:
attempting to show a radical reaction, not knowing how bromine adds to alkenes.
B2
LiN(iPr)2 is LDA, a strong base (pKa = 35), which will form an enolate of the ester. This enolate will then react with the ketone (similar to the aldol reaction). The product of this step contains an alkoxide and there is a good leaving group (the chlorine) on the adjacent carbon so an intramolecular Williamson type reaction - just SN2 - happens to form a cyclic ether, the epoxide (note : this last step is also like using a halohydrin to form an epoxide)
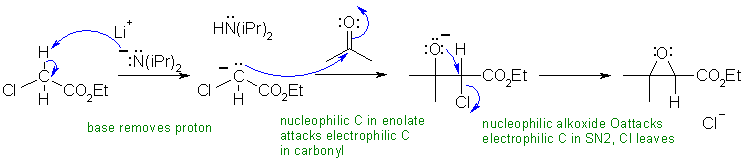
Common errors in this scheme included not recognising the strong base (It's LDA) and the source of it's electrons.
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. vague (i.e. starting / ending "in space" (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) not knowing the basic mechanism types (9) drawing arrows that were inconsistent with the resulting bonding changes.