Answers to the synthesis problems are given below. These answers have been selected as they are short and efficient, but there are probably other reasonable solutions. Note some targets have specific stereochemistry that needs to be considered.
When planning syntheses, one should try to work backwards by functional group analysis. The diagrams show the "retrosynthesis" - the design or plan and then below that the reaction scheme step-by-step (as required in the question). Red arrows try to show carbon-carbon bond forming reactions, blue arrows are functional group manipulations. The blue text rationalise the retrosynthetic analysis and information about important reactions are given in green.
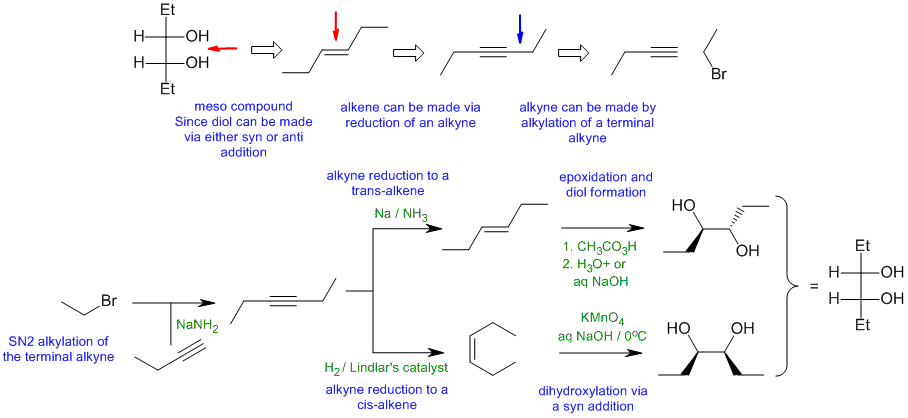
A1

Common errors:
dealing with the required stereochemistry, incorrect reagents for forming the diol
A2
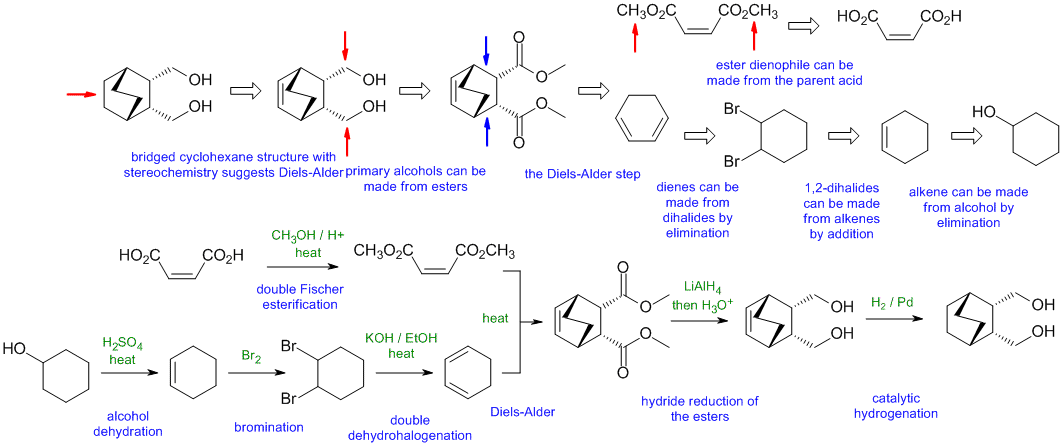
Common errors:
poor choice of dienophile, wrong Diels-Alder reaction
B1
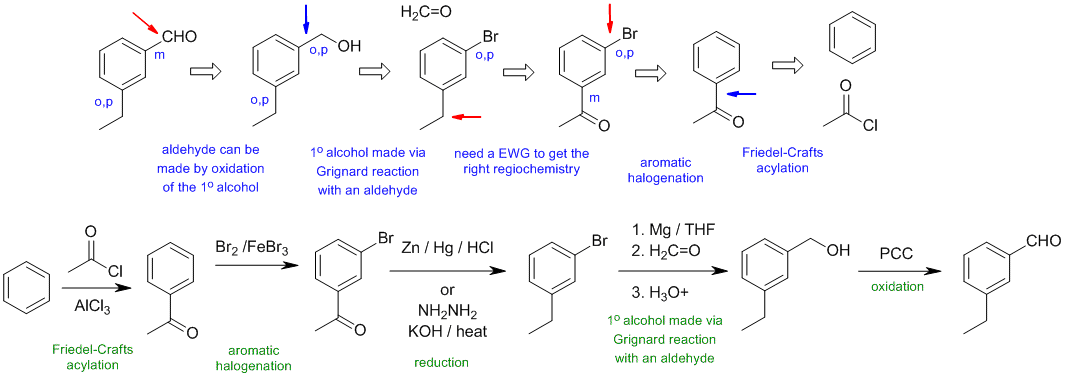
Common errors:
trying to make the aldehyde directly via Friedel-Crafts acylation, regiochemistry
B2
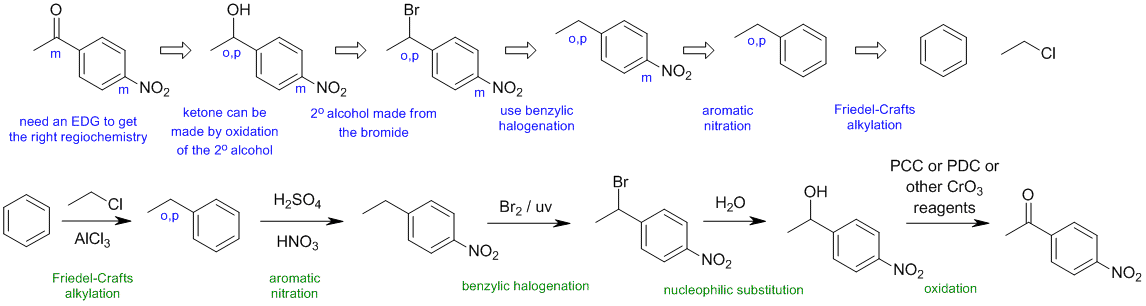
Common errors:
trying to do Friedel-Crafts on a deactivated system, regiochemistry
C1
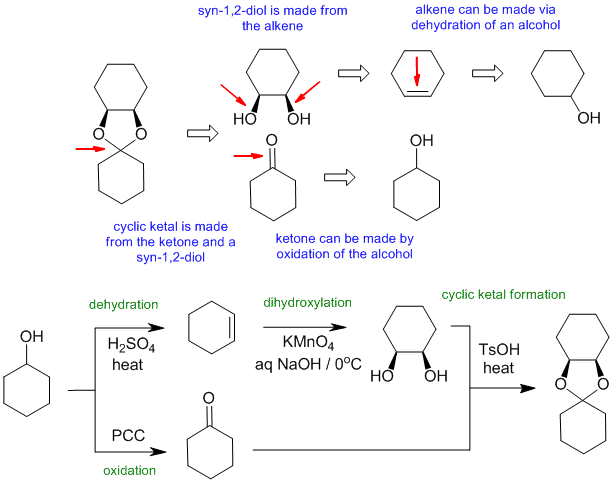
Common errors:
incorrect reagents for alcohol dehydration and diol formation
C2
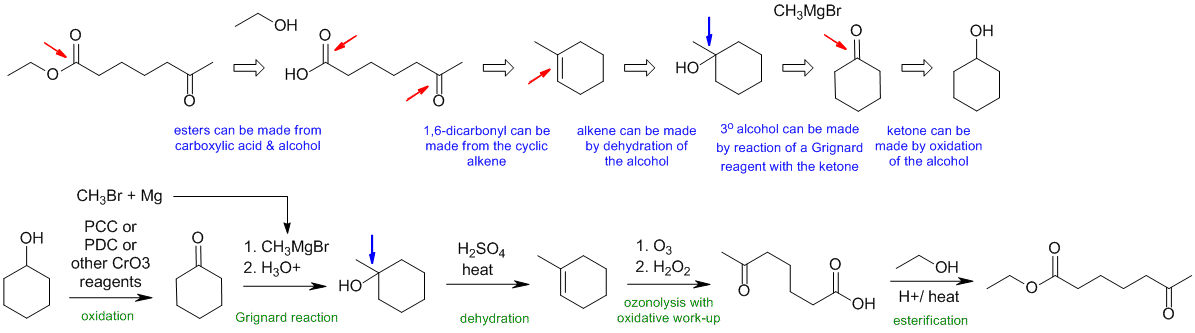
General comments.... Some students started from hydrocarbons with 3 C or less and then made errors in making the materials they could have just used as starting materials.
Common errors included:
(1) incorrect counting of C atoms
(2) trying Freidel-Crafts reactions on deactivated systems or with vinyl halides or aryl halides or ignoring rearrangements in alkylations
(3) trying to use Friedel-Crafts to make benzaldehyde (it can only be used to give aromatic ketones)
(4) trying to do Grignard reactions in the presence of acidic H atoms (e.g. -OH / -NH or CO2H groups)
(5) trying to use Grignard reactions of RMgX with another R-X in an attempt to make R-R systems (these tend to fail due to elimination or Mg exchange).
(6) trying to make Grignard reagents from R-H rather than R-X (X = Cl, Br or I)
(7) poor management / control / incorrect regio and/or stereochemistry
(8) pKa issues....i.e. using strong nucleophiles (i.e. strong bases) in the presence of acidic groups.