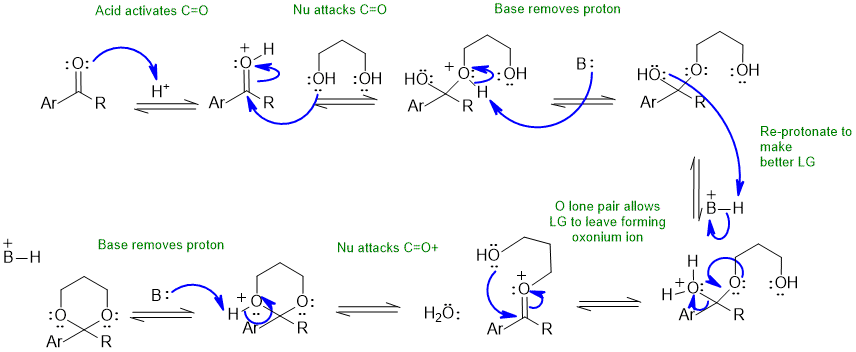
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
A
Ketal formation (forwards):

Ketal hydrolysis (backwards):
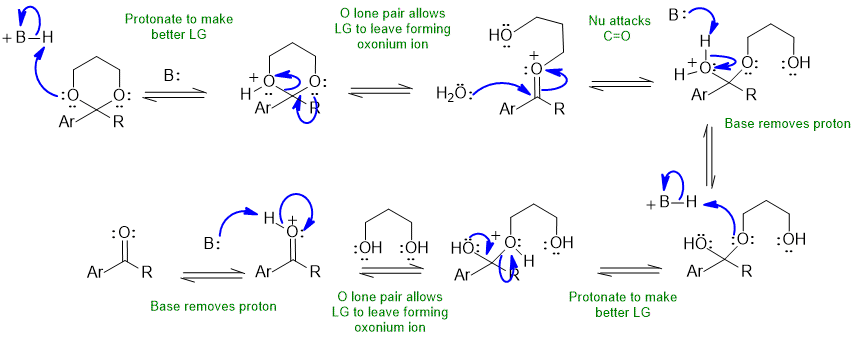
In these schemes Ar and R have been used to represent the aromatic and alkyl substitutents of the ketone (to help keep the scheme looking "cleaner".
The base B: could be the conjugate base of the acid catalyst, carbonyl group, water, diol etc.
Common errors : not using the H+ catalyst to activate the carbonyl (after all we have a neutral and hence weak O nucleophile), not protonating "O" groups to make better leaving groups, using RO- species despite the acid conditions.
B1 Aromatic nitration :
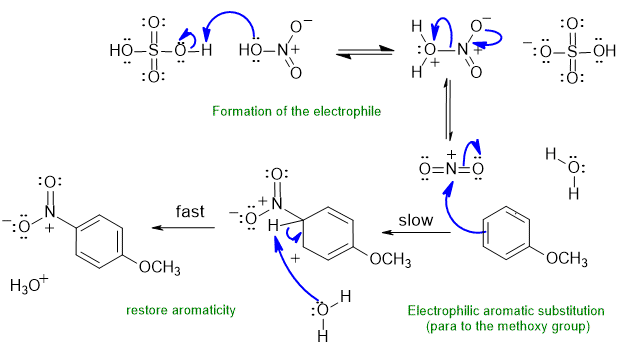
Common errors : (1) incorrect structures of nitro group or sulfuric acid (2) didn't form the E+ correctly (3) formal charges incorrect and (4) loss of the wrong H or not removing an H at all to restoring aromaticity
B2 Aromatic alkylation :
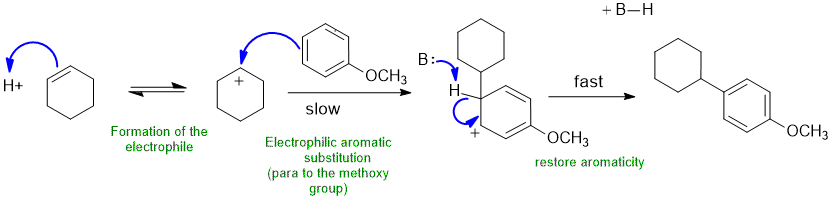
Common errors : (1) didn't form the E+ correctly (2) formal charges incorrect and (3) loss of the wrong H or not removing an H at all to restoring aromaticity
In this scheme, the B could be the conjugate base of the acid catalyst, or the C=C.