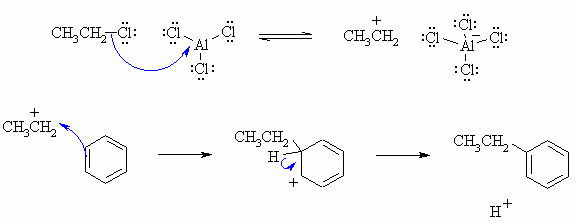
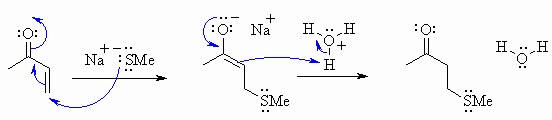
The following diagram show the answers to the mechanistic questions. Note how the curly arrows are drawn specifically to lead from one structure to the next in the sequence for each question. Also check to see how the arrows show how the electrons move to account for all the bonding changes that occur. There was no need to add extra bonds or compounds to complete the sequences. Remember to balance your charges !


| The second part of the section required you to apply the
general principles
of drawing mechanisms to suggest how urea may be formed by heating
ammonium
isocyanate.
Note that isocyanate can be viewed as a carbonyl containing system and that comparing the isocyanate with urea, we can see that we need to make a new C-N bond, so the NH3 is the Nu. Provided that you can draw a reasonable Lewis structure, you should be able to get the two resonacne forms. Thus protonation to activate the C=O towards the weak Nu, NH3, is followed by a series of acid / base protonation / deprotonatioin steps to get to the UREA. |
 |