| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
Lewis Diagrams Answers
1. The compounds are H2, BH3, CH4,
NH3, H2O and HF:
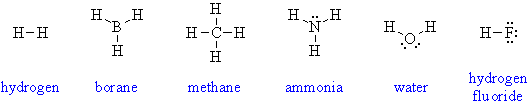
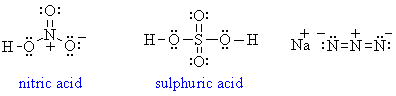
2. Draw Lewis diagrams for the following inorganic reagents: HNO3, H2SO4, and sodium azide.
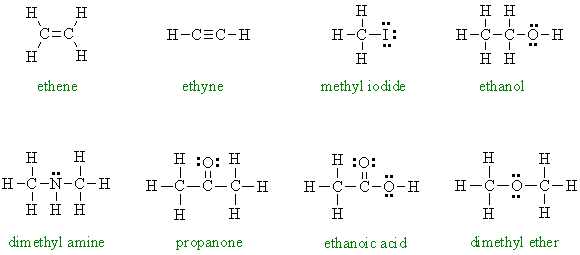
3. The Lewis diagrams for the molecules are shown (these were selected to represent important classes of organic molecules that you will be seeing a lot of in the future : note it is very important to know where the lone pairs are in the structures). Note that none of these structures have any formal charges and all the C, N, O (and I) atoms have complete octets.