| Useful Concepts |
| Useful Concepts |
| Let's look at the curly arrows for some NUCLEOPHILIC
SUBSTITUTION reactions.
Overall, the processes involved are quite similar to those for the ACID / BASE reactions described in lesson 2. Overall in a nucleophilic substitution, a nucleophile (Nu) becomes bonded to a carbon atom and a leaving group (LG) is displaced. In bonding terms, this means we must MAKE a Nu-C bond and break a C-LG bond. 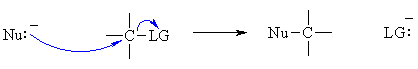 Consider the stepwise version of the reaction (an SN1 reaction), for
example the reaction of t-BuCl with HO-. Before we can
make a new bond to the central C atom, we must first break the C-Cl bond
by removing the electrons from the bond and giving them to the more electronegative
Cl atom. This is just an extension of the polar character of the
C-Cl covalent bond.
Since we have taken electrrons AWAY from C it becomes a +ve carbocation and since we GIVE electrons to Cl it becomes the -ve chloride ion. Why doesn't the Cl have a -2 charge since it gained 2 electrons ? Do the charges balance ? Yes, we have a neutral molecule going to +ve and -ve whose charges cancel. In the second step, the electron rich nucleophile donates the electrons to form a new C-O bond. Note the curly arrow starts from where the electrons are, the lone pairs on the oxygen atom, and goes from electron rich to electron poor:
Do the charges balance ? Yes, we have a +ve ion reacting with a -ve ion to give a neutral molecule. Now let's consider the single step version of the reaction (an SN2 reaction),
for example the reaction of CH3Cl with HO-, where
the bond making and breaking occur simultaneously.
|