The Periodic Table
| s-block d-block p-block | |||||||||||||||||
|
H |
He* |
He* |
|||||||||||||||
|
Li 6.94 |
Be 9.01 |
B 10.8 |
C 12.0 |
N 14.0 |
O 16.0 |
F 19.0 |
Ne 20.2 |
||||||||||
|
Na 23.0 |
Mg 24.3 |
Al 27.0 |
Si 28.1 |
P 31.0 |
S 32.1 |
Cl 35.5 |
Ar 40.0 |
||||||||||
|
K 39.1 |
Ca 40.1 |
Sc 45.0 |
Ti 47.9 |
V 50.9 |
Cr 52.0 |
Mn 54.9 |
Fe 55.8 |
Co 58.9 |
Ni 58.7 |
Cu 63.5 |
Zn 65.4 |
Ga 69.7 |
Ge 72.6 |
As 74.9 |
Se 79.0 |
Br 79.9 |
Kr 83.8 |
|
Rb 85.5 |
Sr 87.6 |
Y 88.9 |
Zr 91.2 |
Nb 92.9 |
Mo 95.9 |
Tc 98 |
Ru 101 |
Rh 103 |
Pd 106 |
Ag 108 |
Cd 112 |
In 115 |
Sn 119 |
Sb 122 |
Te 128 |
I 127 |
Xe 131 |
|
Cs 133 |
Ba 137 |
La* 139 |
Hf 178 |
Ta 181 |
W 184 |
Re 186 |
Os 190 |
Ir 192 |
Pt 195 |
Au 197 |
Hg 201 |
Tl 204 |
Pb 207 |
Bi 209 |
Po 210 |
At 210 |
Rn 222 |
|
Fr 223 |
Ra 226 |
Ac* 227 |
Ku |
Ha |
|
||||||||||||
Notes:
*He, Helium is typically positioned top right in the Periodic Table because it's a noble gas and "belongs" with the other noble gases such as neon and argon. Recall that the noble gases are inert because they are stable which is in turn because they have complete shells. For helium, it's the 1s shell that is full, for the others, it's the p orbital set that is full. That's way in this context, it's fair to put helium on the left side next to hydrogen.
How to use the Periodic Table to determine the order of orbital filling
1. Start from the top left with hydrogen
2. Read across the rows, in order of increasing atomic number.
3. For each element, the colour of the block indicates which orbitals are being filled. This allows you to read off the order of the orbitals....this means the orbital energies list from lowest (most stable) to highest (least stable) and hence the orbital filling order is 1s 2s 2p 3s 3p 4s 3d 4p etc.
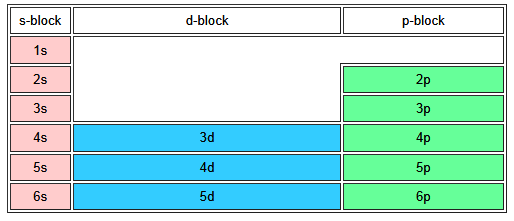
(this image ignores the lanthanides and actinides in the f-block series)