| Chapter 2: Bonding |
| Chapter 2: Bonding |
Bonding in H2
In Chapter 1 we introduced the idea that electrons have wave-like properties that led to the concept of electrons being in atomic orbitals (review ?) But we also need to consider what happens to the electrons and the associated atomic orbitals when a molecule forms.
Here we are going to consider what happens as two hydrogen atoms come together to form a molecule of hydrogen:
![]()
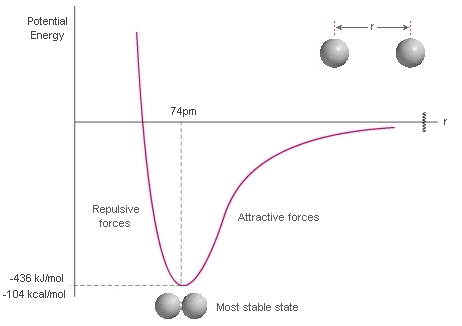 |
First, let's consider what happens to the energy of two H atoms as they get closer to each other from infinite separation. The curve represents the energy (y axis) of the system as the distance "r" between the two atoms changes (x axis). 1. At inifinite separation (r = ∞) the two atoms are assumed to be far enough apart that they are unaffected by each other. This means we have individual atoms. 2. As the atoms move towards each other from infinite separation (move left towards zero on the x axis), there is an initial attraction of the electrons of one atom to the nuclei of the other atom (opposite charges, attractive forces). This stabilises the system (energy decreases, y axis). 3. At some point however, the atoms get so close to each other that the two nuclei (like charges) start to repel each other (repulsive forces), there is destablisation and the energy increases (y axis). Key values: The most stable state (lowest energy, minimum on y axis) corresponds to the equilibrium bond length. For H-H this is 74 pm (0.74 Angstroms). The energy difference between the most stable state and the energy at infinite separation (zero by definition) represents the energy that needs to be put in to the system (i.e. required) to take an H-H molecule to two separate atoms. This corresponds to the energy required to break the bond and hence the bond strength. For H-H this is 436 kJ/mol (or 104 kcal/mol). Of course, if we look at it in the opposite sense, it is the energy released when two separate atoms of H come together to form a molecule of H-H. |
 |
© Dr. Ian Hunt, Department of Chemistry |