| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
Hybridisation
Hybridisation is a concept that
allows us to account for certain
key structural issues that are not easily accounted for in other
bonding
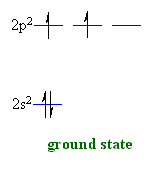
theories when starting from the ground state of a carbon atom 2s2
2p2.
The concept involves the "cross breeding" of atomic orbitals to create
"new" orbitals. Hence the use of the term "hybrid" : think of a
hybrid
animal which is a cross breed of two species.
| STUDY TIP :
It may be useful to think of hybridisation like a food blender
!
In the kitchen, take 1 orange and 3 apples, put them in a food blender, put the lid on (so nothing comes out), and turn on the blender. When you stop you have a fruit mixture made from 4 pieces of fruit that has characteristics of both apples and oranges. At the atomic level, take 1 x 2s orbital and 3 x 2p orbitals, put them in a "blender", put the lid on (so nothing comes out), and turn on the "blender". When you stop you have an orbital mixture made from 4 orbitals that has characteristics of both s and p orbitals. |
Let's pose the bonding problems in the context of
methane, CH4
|
 |
General
method
|
|
|
 |
|
 |
| * This simplification has to be modified if there is a resonance interaction with another group, e.g. in amides . | |
| © Dr. Ian Hunt, Department of Chemistry, University of Calgary |