| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
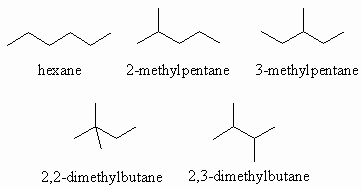
Ans 1 : The molecular formula of C6H14 contains the maximum number of H atoms for 6C (CnH2n+2) so it can cannot contain double bonds or rings. Therefore the only functional group that can be present is an alkane. When you draw them it is a good idea to have a system whereby you start with the longest chain, then move to shorter chains with an increasing number of substitutents, moving them to different positions on the chain as you go. Watch that you don't repeat structures.
Ans 2 : The molecular formula of C5H10 contains 2 less H atoms than pentane (C5H12) so that it must contain either a double bond or one ring. Therefore in terms of functional groups we need to consider alkenes and cycloalkanes.
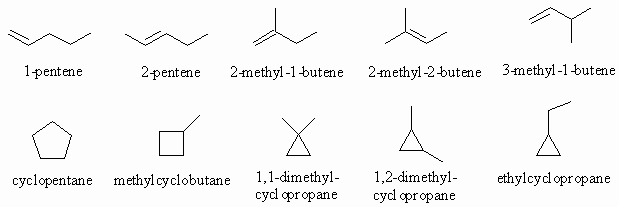
Ans 4 : In order to answer the question, it is a very good idea to draw a diagram that relates the following three equations that can be use to create the Hess's Law "triangle" that relates the alkanes, their component elements and their combustion products.
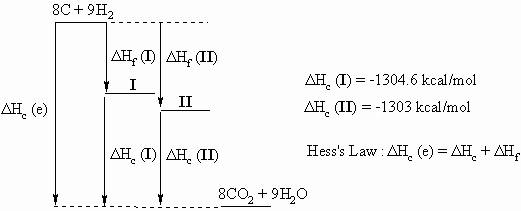
| Reaction | ?H | Eqn |
| C8H18 ----------> 8 CO2 + 9 H2O | Combustion (known), ΔHc(alkane) | 1 |
| 8 C + 9 H2 -----> 8 CO2 + 9 H2O | Combustion (calculate), ΔHc(elements) | 2 |
| 8 C + 9 H2 ----------> C8H18 | Formation (required) ΔHf(alkane) | 3 |
From Hess's Law we get that ΔHf (alkane) = ΔHc(e) - ΔHc(alkane)
ΔHc(e)= 8 x ΔHc(C) + 9 x ΔHc(H2) = 8 x -94.05 + 9 x -57.8 = -1272.6 kcal/mol.
So for 2,2-dimethylhexane, I, ΔHf = -1272.6 - - 1304.6 = 32 kcal/mol
and for 2,2,3,3-tetramethylbutane, II, ΔHf = -1272.6 - -1303.0 = 30.4 kcal/mol
Thus 2,2,3,3-tetramethylbutane is the more
stable since it has the more
exothermic ΔHf. (care ! both
are calculated to be endothermic). This is also evident in that II
has the less exothermic ΔHc, and is the most branched of the two
hydrocarbons.
The relative stability is most clearly demonstrated and appreciated by
the diagram, otherwise be very cautious with the signs of your
calculations.
| © Dr. Ian Hunt, Department of Chemistry, University of Calgary |