| Chapter 4: Resonance |
| Chapter 4: Resonance |
Why do we need Resonance ?
We can't see molecules which means we can not photograph them so we have to create representations of them. The most common way for us to do that is to draw images of a molecule and Lewis structures are the true basis of the structures we would create. A key feature of Lewis structures is that they require that we show bonding and non-bonding electrons in specific locations (i.e. the electrons are localised).
Some molecules that contain π systems can be represented by more than one Lewis structure where the only difference is the location of π electrons (i.e. the σ bond frameworks are identical).
Electrons in σ bonds have a fixed location and so they are said to be localised.
In contrast, some π electrons that can be drawn in different locations are said to be delocalised.
This type of situation raises questions such as which of these is the best (i.e. most accurate) representation of the structure.
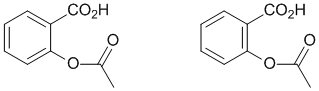
As an example, consider the compound aspirin (shown above). Careful inspection shows that there is a slight difference. Can you spot the difference ? ANSWER
So which of these two best represents the actual structure ? The answer to that is they are essentially equally valid, but neither is quite right ! We will come back soon to discuss this in more detail.
Ozone
Benzene
Therefore, resonance is a tool that chemists (especially organic chemists) need to use to help deal with implications of having a molecule that can not be simply represented by a single Lewis structure. Resonance helps us deal with the situation where the true, real structure of a molecule is somewhere between two (or more) Lewis structures.
In particular, Lewis structures are useful when we try to create representations of chemical reactions and draw arrows to show the movement of electrons. In order to do this, one really needs to use structures that show the electrons in specific locations (i.e. they are localised). It is much more difficult to show what the electrons are doing if the location of the electrons aren't fixed (i.e. they are delocalised).
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |