Chapter 6: Acidity and Basicity |
Chapter 6: Acidity and Basicity |
![]()
Derivation .... start from the definition of Ka, take -log10 of both sides and substitute in for definition of pH.
Applications.
1. Determination of pKa
2. Quantitative : Calculation of the ratio of [A-] : [HA] for a solution of known pH if the pKa for the acid dissociation is known
3. Qualitative : Prediction of the major species present in a solution of a known pH if the pKa values are known
e.g.
a. if a compound with pKa = 5 is present in a solution of pH = 3, then the [HA] : [A-] = 100 (since log10 [HA] / [A-] = 2). Note that this corresponds to roughly a 99% : 1% ratio
(since the solution is more acidic than the pKa therefore the compound exists mainly in the acid (protonated) form)
b. if a compound with pKa = 5 is present in a solution of pH = 6, then the [HA] : [A-] = 0.1 (since log10 [HA] / [A-] = -1)
(since the solution is more basic than the pKa therefore the compound exists mainly in the basic (deprotonated) form)
Example
This tells us that when the pH = pKa then log [HA] / [A-]
= 0 therefore [HA] = [A-] ie equal amounts of the two forms.
If we make the solution more acidic, ie lower the pH, then pH < pKa and log [HA] / [A-] has to be > 0 so [HA] > [A-].
This makes sense as it tells us that the protonated form dominates in an acidic
medium.
If instead we make the solution more basic, ie raise the pH, then pH
> pKa and log [HA] / [A-] has to be < 0 so [HA]
<[A-]. This makes sense as it tells us that the deprotonated form
dominates in the basic medium.
These principles can be extended to poly acidic / basic systems (such as amino acids) by considering each pKa value in turn.
Lets look at an example.
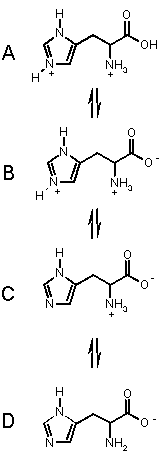
To the right are the processes for the amino acid
HISTIDINE, which has three acidic groups of pKa's 1.82 (carboxylic
acid) 6.04 (pyrrole NH) and 9.17 (ammonium NH). Histidine can exist in the four
forms shown, depending on the solution pH, from acidic pH (top) to basic pH.
(bottom).
Starting from the top, we can imagine that as we add base, the most acidic proton
is removed first (COOH), then the pyrrole NH then finally the amino NH. These
takes us through each of the forms in turn.
At pH < 1.82, A is the dominant form.
In the range 1.82 < pH < 6.02 B is the dominant form.
In the range 6.02 < pH < 9.17 C is the dominant form, and when pH > 9.17, D is the major form in solution. OK?
 |
© Dr. Ian Hunt, Department of Chemistry |