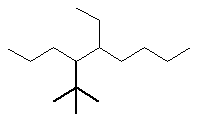
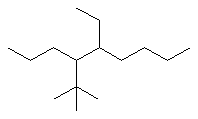
Now work on the whole structure:
- Functional group is an alkane, therefore suffix = -ane
- The longest continuous chain is C9 therefore root = non
- There are two substituents:
- simple substituent is a C2 alkyl group i.e. an ethyl group
- a complex substituent (see above) = (1,1-dimethylethyl)
- The first point of difference rule requires numbering from the left
as drawn, the locants are 4-
and 5-
- Alphabetisation requires that the complex substituent be listed
first since "d" is before "e"
4-(1,1-dimethylethyl)-5-ethylnonane
|
|
|