 |
|
 |
|
(1) Substituted systems The ring systems have no common atoms. In naming, it should be treated as a subsituted cycloalkane where the smaller ring is regarded as a substituent of the larger ring. The simplest example is the C6 system shown to the right.
|
||
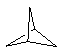
| (2) Spiro ring systems Spiro ring systems share a single common atom. Hence the rings join at a single "point". The simplest example is the C5 system shown to the right.
|
|
|
| (3) Fused ring systems Fused ring systems share two common atoms in one common bond, hence the rings share one side. The simplest polycyclic system is the C4 system shown to the right where the rings share two atoms (one common side).
|
|
|
| (4) Bridged ring systems Bridged ring systems share more than two common atoms. The simplest polycyclic system is the C5 system shown to the right where the rings contain three common atoms (two common sides). If we view the two rings as been the one on the left and the other on the right, then the highlighted C atoms are common to both (mouseover image).
|
|
| ©Dr. Ian Hunt, Department of Chemistry |