 |
Basic IUPAC Organic Nomenclature
|
 |
Basic IUPAC Organic Nomenclature
|
E- and Z-nomenclature of alkenes
On the previous pages, we looked at naming alkenes as cis- and trans-.
Misconception Alert! cis ¹ Z and trans ¹ E In general terms there is NO specific relationship between cis and trans / E and Z as they are based on fundamentally different naming rules. |
It is important to note that the two methods are different (i.e. they are based on different rules) and
they are NOT interchangeable, see below for an example.
The cis- / trans- style is based on the longest chain whereas the
E/Z style is based on a set of priority rules.
You need to know both styles.
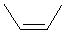 |
 |
| cis-but-2-ene or (Z)-but-2-ene |
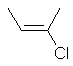
cis-2-chlorobut-2-ene
or (E)-2-chlorobut-2-ene |
The E- and Z- style is more reliable and particularly suited to tri- or tetra-substituted
alkenes, and especially when the substituents are not alkyl groups.
The Cahn-Ingold-Prelog priority rules are used for naming geometric isomers (e.g. E- or Z-alkenes) and
other stereoisomers (see later).
These rules are based on atomic number, and the first point of difference.
 |
 |
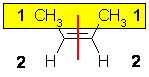 |
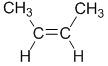
| Step 1 : split the alkene |
Step 2 : assign the relative priorities. The two attached atoms are C and H, so since the atomic numbers C > H then the -CH3 group is higher priority. |
Step 3 : look at the relative positions of
the higher priority groups : same side = Z, hence (Z)-but-2-ene. |
 |
|
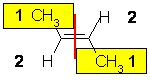
| The two attached atoms are C and H, so since the atomic numbers C > H then the -CH3 group is higher priority. Therefore the two high priority groups are on the opposite side, then this is (E)-but-2-ene. | |
Subrules:
| ©Dr. Ian Hunt, Department of Chemistry |