 |
|
 |
|
| Nomenclature |
Formula |
3D structure |
|
Functional class name = alkylamines or alkanamines
|
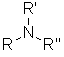 |
|
butan-2-amine or 2-butylamine
(or sec-butylamine) |
CH3CH2CH(NH2)CH3 |
|
N-methylethylamine
or N-methylethanamine |
CH3NHCH2CH3 |
|
Name : N,N-dimethylmethylamine
or N,N-dimethylmethanamine or trimethylamine |
(CH3)3N |
| ©Dr. Ian Hunt, Department of Chemistry |