 |
|
 |
|
| Nomenclature |
Formula |
3D structure |
| Functional class name = alkyl
alkyl ether e.g. ethyl methyl ether Substituent prefix = alkoxy- e.g. methoxyethane |
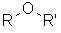 |
|
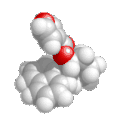
ethyl methyl ether
|
CH3CH2OCH3
|
|
|
CH3CH2OCH2CH3 |
|
1-methoxypropane
|
CH3CH2CH2OCH3
|
|
2-methoxypropane
|
CH3CH(OCH3)CH3
|
|
CH3OCH2CH2CH3 | |
| ©Dr. Ian Hunt, Department of Chemistry |