 |
Basic IUPAC Organic Nomenclature
|
 |
Basic IUPAC Organic Nomenclature
|
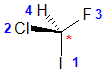
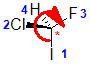
| The most common chirality center in organic chemistry is a carbon atom with four different groups attached |
|
|
The chirality center should be easy to spot, and the four attached groups are in priority order, highest to to lowest: I (purple), Cl (green), F(brown) and H (white) Rotate the image on the left so the you are looking along the C-H bond
and the H is away from you, then determine the direction of high
to low priority. |
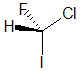 |
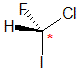 |
 |
 |
 |
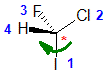
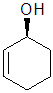
| starting point | identify the chirality center(s | assign the relative
priorities then rotate the low
priority group away (to the back) |
determine the sense
of groups 1 - 3 clockwise = R |
 |
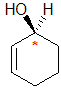 |
 |
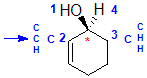 |
|
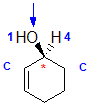
| starting point |
identify the chirality center(s) and show the implied H atom |
assign the relative
priorities.... Because we have two C groups we need to list the groups
the C are attached to in atomic number order |
the first point of
difference is the C > H so the C group on the left is the higher priority |
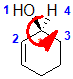
with the low priority group already at the back, determine the
sense of groups 1 - 3 counterclockwise = S |
| ©Dr. Ian Hunt, Department of Chemistry |