| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
Reactions of Alcohols to give Acetals
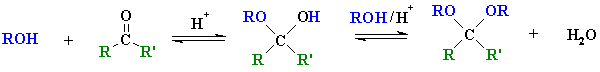 |
| hemi-acetal acetal |
Reaction type: Nucleophilic Addition then nucleophilic substitution
Summary
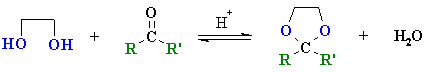
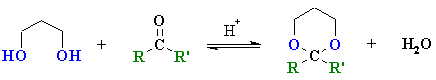
| Study Tip: The important "piece" of an acetal is the central C which becomes the C of the carbonyl C=O. It can be recognised by looking for the C that is attached to two O atoms by single bonds. |
Related Reactions
MECHANISM
FOR THE ACID CATALYSED FORMATION OF ACETALS |
|
| Step 1: An acid/base reaction. Since there is only a weak nucleophile we need to activate the carbonyl by protonating on O. |
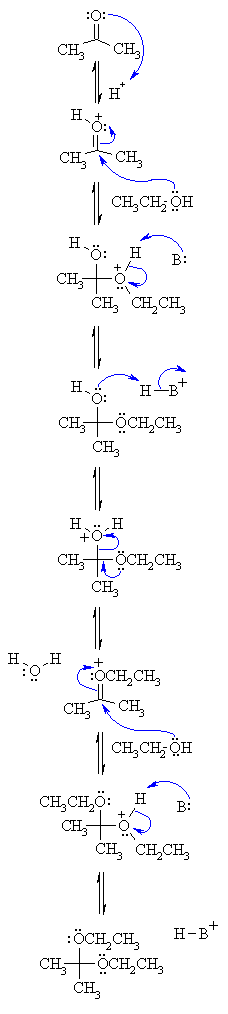 |
| Step 2: The nucleophilic O in the alcohol attacks the electrophilic C in the C=O, breaking the π bond and giving the electrons to the positive O. |
|
| Step 3: An acid/base reaction. Deprotonation of the alcoholic oxonium ion neutralises the charge giving the hemi-acetal. Now we need to substitute the -OH by -OEt. |
|
| Step 4: An acid/base reaction. In order for the -OH to leave we need to make it into a better leaving group by protonation. |
|
| Step 5: Using the electrons from the other O, the leaving group departure is facilitated. |
|
| Step 6: We now have what resembles a protonated ketone (compare with step 2). The nucleophilic O of the alcohol attacks the electrophilic C and the electrons of the π bond move to neutralise the charge on the positive O. |
|
| Step 7: An acid/base reaction. Deprotonation of the alcoholic oxonium neutralises the charge and produces the acetal product and regenerates the acid catalyst. |
|
| © Dr. Ian Hunt, Department of Chemistry |