 |
Chapter 13: Spectroscopy |
 |
Infra-Red (IR) Spectroscopy
Basics:
Infra red (IR) spectroscopy deals with the interaction
between a molecule and radiation from the IR region of the EM spectrum (IR region
= 4000 - 400 cm-1). The cm-1 unit is
the wave number scale and is given by 1 / (wavelength in cm).
IR radiation causes the excitation of the vibrations
of covalent bonds within that molecule. These vibrations include the stretching
and bending modes.
An IR spectrum show the energy absorptions as one
'scans' the IR region of the EM spectrum. As an example, the IR spectrum
of butanal is shown below.
In general terms it is convienient to split an
IR spectrum into two approximate regions:
- 4000-1000 cm-1 known as the functional group region,
and
- < 1000 cm-1 known as the fingerprint region
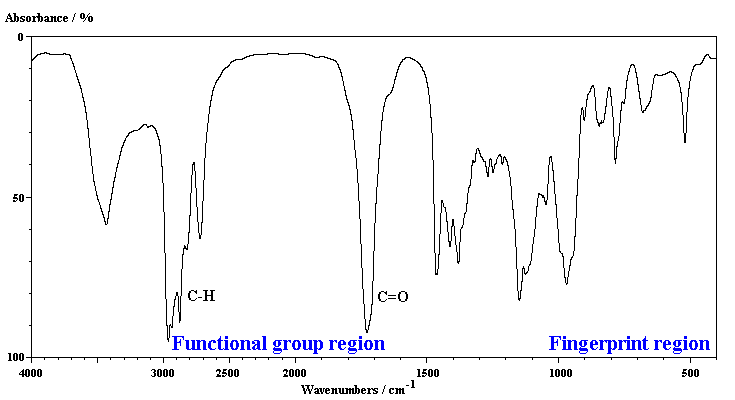
- Most of the information that is used to interpret an IR spectrum is obtained
from the functional group region.
- In practice, it is the polar covalent bonds than are IR "active"
and whose excitation can be observed in an IR spectrum.
- In organic molecules these polar covalent bonds represent the functional
groups.
- Hence, the most useful information obtained
from an IR spectrum is what functional groups are present within the molecule
(NMR spectroscopy typically gives the hydrocarbon fragments).
- Remember that some functional groups can be "viewed" as combinations of
different bond types. For example, an ester, CO2R contains
both C=O and C-O bonds, and both are typically seen in an IR spectrum of an
ester.
- In the fingerprint region, the spectra tend to be more complex
and much harder to assign.
|
MOST IMPORTANT THING TO REMEMBER.....
When analysing an IR spectrum avoid the temptation to try to assign every peak.
The fingerprint region, however, can be useful for helping to
confirm a structure by direct comparison with a known spectra.
|
