| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Basic Principles
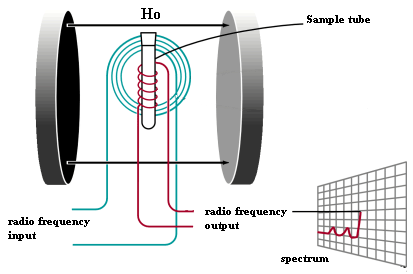 |
The basic arrangement of an NMR spectrometer is shown to the left. The sample is positioned in the magnetic field and excited via pulsations in the radio frequency input circuit. The realigned magnetic fields induce a radio signal in the output circuit which is used to generate the output signal. Fourier analysis of the complex output produces the actual spectrum. The pulse is repeated as many times as necessary to allow the signals to be identified from the background noise. |
| © Dr. Ian Hunt, Department of Chemistry |