| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Nuclear Magnetic Resonance (NMR) Spectroscopy
Since a nucleus is a charged particle in motion,
it will develop a magnetic field. 1H and 13C
have nuclear spins of 1/2 and so they behave in a similar fashion to a simple,
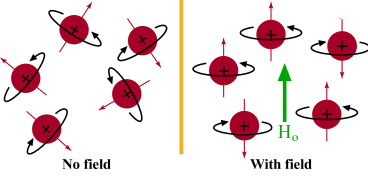
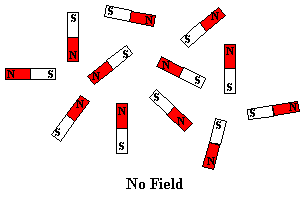
tiny bar magnet. In the absence of a magnetic field, these are randomly
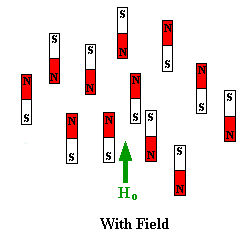
oriented but when a field is applied they line up parallel to the applied field,
either spin aligned or spin opposed. The more highly populated state
is the lower energy spin state spin aligned situation. Two schematic
representations of these arrangements are shown below:
| © Dr. Ian Hunt, Department of Chemistry |