| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Shielding in H-NMR
The various factors that influence the field include:
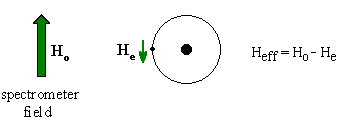
ElectronegativityThe electrons around the proton create a magnetic
field that opposes the applied field. Since this reduces the field
experienced at the nucleus, the electrons are said to shield the
proton. It can be useful to think of this in terms of vectors....
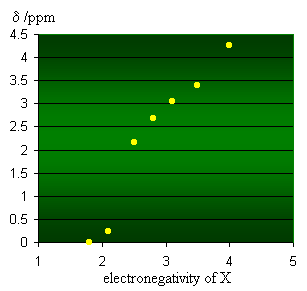 |
Since the field experienced by the proton defines the energy difference between the two spin states, the frequency and hence the chemical shift, δ /ppm, will change depending on the electron density around the proton. Electronegative groups attached to the C-H system decrease the electron density around the protons, and there is less shielding (i.e. deshielding) so the chemical shift increases. This is reflected by the plot shown in the graph to the left which is based on the data shown below. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| These effects are cumulative, so the presence of more electronegative groups produce more deshielding and therefore, larger chemical shifts. |
|
| These inductive effects are not just felt by the immediately adjacent protons as the disruption of electron density has an influence further down the chain. However, the effect does fade rapidly as you move away from the electronegative group. As an example, look at the chemical shifts for part of a primary bromide |
|
The word "anisotropic" means "non-uniform". So magnetic anisotropy means that there is a "non-uniform magnetic field". Electrons in π systems (e.g. aromatics, alkenes, alkynes, carbonyls etc.) interact with the applied field which induces a magnetic field that causes the anisotropy. As a result, the nearby protons will experience 3 fields: the applied field, the shielding field of the valence electrons and the field due to the π system. Depending on the position of the proton in this third field, it can be either shielded (smaller d) or deshielded (larger d), which implies that the energy required for, and the frequency of the absorption will change.
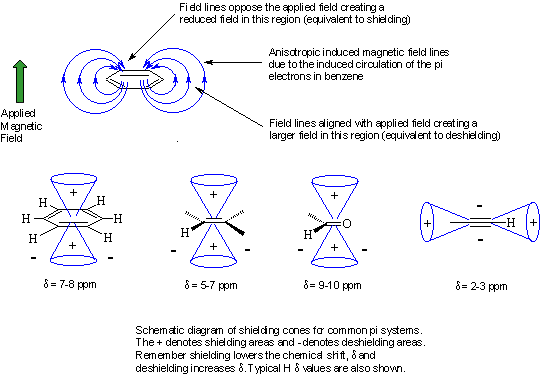
Protons that are involved in hydrogen bonding (this usually means -OH or -NH) are typically observed over a large range of chemical shift values. The more hydrogen bonding there is, the more the proton is deshielded and the higher its chemical shift will be. However, since the amount of hydrogen bonding is susceptible to factors such as solvation, acidity, concentration and temperature, it can often be difficult to predict.
HINT : It is often a good idea to leave assigning -OH or -NH resonances until other assignments have been made.
Experimentally, -OH and -NH protons can be identified by carrying out a simple D2O (deuterium oxide, also known as heavy water) exchange experiment. These H atoms are said to be exchangeable.
Consider the alcohol case for example:
R-OH + D2O <=>
R-OD + HOD
During the hydrogen bonding, the alcohol and heavy water can "exchange" -H
and -D each other, so the alcohol becomes R-OD.
Although D is NMR active, its signals are of different energy and are
not seen in the H-NMR, hence the peak due to the -OH disappears.
(Note that the HOD will appear...)
QUESTION
| © Dr. Ian Hunt, Department of Chemistry |