| Chapter 15: Alcohols, Diols and Thiols |
| Chapter 15: Alcohols, Diols and Thiols |
Reductions of Carboxylic Acids and Esters
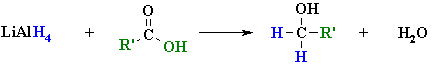
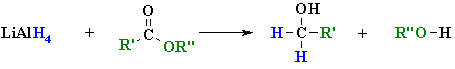
Summary
| REACTION OF
LiAlH4 WITH AN ESTER |
|
| Step 1: The nucleophilic H from the hydride reagent adds to the electrophilic C in the polar carbonyl group of the ester. Electrons from the C=O move to the electronegative O creating an intermediate metal alkoxide complex. |
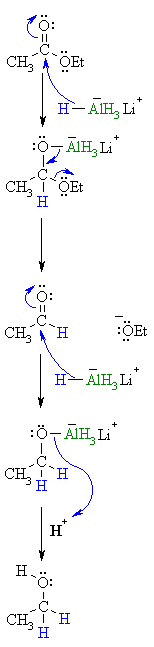
|
| Step 2:
The tetrahedral intermediate collapses and displaces the alcohol portion of the ester as a leaving group, this produces a ketone as an intermediate. |
|
| Step 3:
Now we are reducing an aldehyde. The nucleophilic H from the hydride reagent adds to the electrophilic C in the polar carbonyl group of the aldehyde. Electrons from the C=O move to the electronegative O creating an intermediate metal alkoxide complex. |
|
| Step 4:
This is the work-up step, a simple acid/base reaction. Protonation of the alkoxide oxygen creates the primary alcohol product from the intermediate complex.
|
|
| © Dr. Ian Hunt, Department of Chemistry |