| Chapter 16: Ethers, Epoxides and Sulfides |
| Chapter 16: Ethers, Epoxides and Sulfides |
Williamson Ether Synthesis
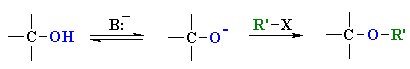
Reaction type: Nucleophilic Substitution (SN2)
Summary
QUESTIONS
| ANSWER | ||
|
|
ANSWER | |
|
|
ANSWER | |
| ANSWER |
Related Reactions
|
|
|
| The alkoxide functions as the nucleophile and attacks the electrophilic C of the alkyl halide displacing the bromide and creating the new C-O bond. |
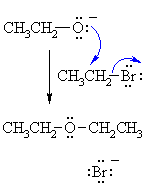 |
| © Dr. Ian Hunt, Department of Chemistry |