| Chapter 16: Ethers, Epoxides and Sulfides |
| Chapter 16: Ethers, Epoxides and Sulfides |
SN1 type Reactions of Epoxides
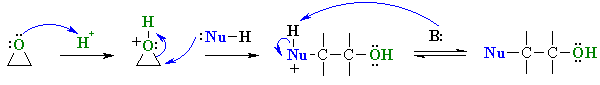
| Scenario 1: The Nu attacks the more substituted C of the epoxide at 180o to the C-O bond that breaks. |
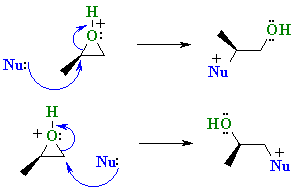 |
| Scenario 2: The Nu attacks the least hindered end of the epoxide at 180o to the C-O bond that breaks. |
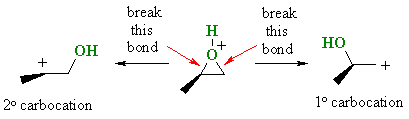
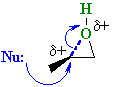 |
In reality it is usually that the epoxide C-O bond to the more substituted center that is weaker resulting in carbocation character at that more substituted C and the nucleophile attacks there.... Stereochemistry ? Because the C-O bond is not totally broken when the nucleophile attacks, the nucleophile still attacks at 180o with respect to the leaving group so there is still inversion at that center. |
| © Dr. Ian Hunt, Department of Chemistry |