| Chapter 19: Carboxylic Acids |
| Chapter 19: Carboxylic Acids |
Carboxylic Acids
Nomenclature:
Functional group suffix = -oic acid (review)
If the acid is substituted onto a ring the suffix -carboxylic acid is
used.
|
|
|
|
|
|
| IUPAC name: | methanoic acid | ethanoic acid | propanedioic acid | butanedioic acid |
| Trivial name: | formic acid | acetic acid | malonic acid | succinic acid |
The anion dervived by deprotonation of a carboxylic acids is the carboxylate.
| Carboxylic Acid | Structure | pKa | |
| Ethanoic acid | CH3CO2H | 4.7 | |
| Propanoic acid | CH3CH2CO2H | 4.9 | |
| Fluoroethanoic acid | CH2FCO2H | 2.6 | |
| Chloroethanoic acid | CH2ClCO2H | 2.9 | |
| Dichloroethanoic acid | CHCl2CO2H | 1.3 | |
| Trichloroethanoic acid | CCl3CO2H | 0.9 | |
| Nitroethanoic acid | O2NCH2CO2H | 1.7 | |
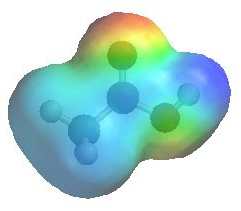 |
The image shows the electrostatic
potential for acetic acid (ethanoic acid). The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density.
|
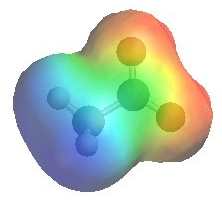 |
The image shows the electrostatic
potential for the acetate ion (ethanoate ion) The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density.
|
| © Dr. Ian Hunt, Department of Chemistry |