|
|
|
|
Structure of Carboxylic Acid Derivatives
|
|
|
|
|
|
|
|
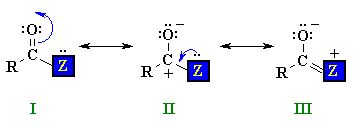
QUESTION What do you notice about the geometry at the N atom ? ANSWER
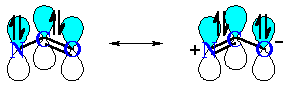
|
|
|
|
|
|
|
|
| © Dr. Ian Hunt, Department of Chemistry |