| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
Nucleophilic Acyl Substitution : Less Reactive Systems
| NUCLEOPHILIC
ACYL SUBSTITUTION FOR LESS REACTIVE SYSTEMS |
|
| Step 1: An acid / base reaction. Protonation of a lone pair on the O of the carbonyl group creates a more electrophilic, cationic system. Step 2: Step 3: Step 4: |
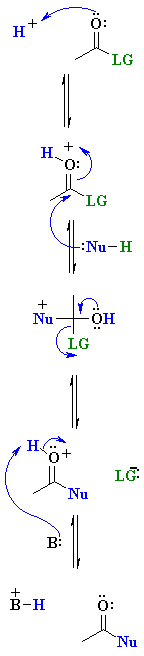 |
Note: In some cases (especially esters and amides) the leaving group must also be activated prior to being lost. This can also be achieved by protonation. This adds a step to the process shown above.
Examples Reactions that follow this mechanism:
| © Dr. Ian Hunt, Department of Chemistry |