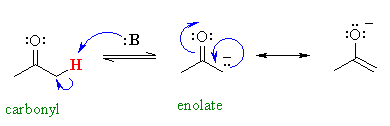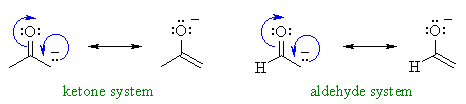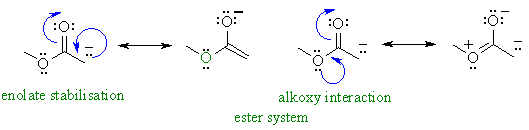 |
Chapter 21: Ester Enolates
|
 |
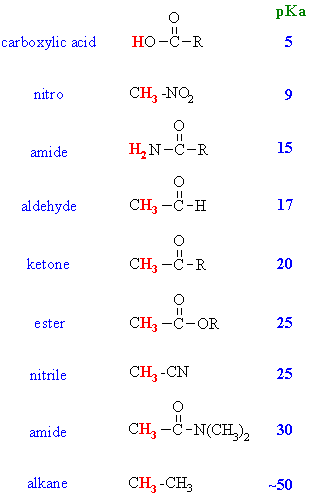
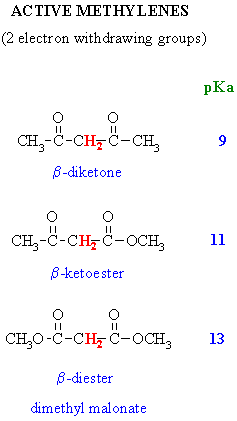
Acidity of a-Hydrogens
- In the following table, the acidity
of the H for various enolate systems and
other closely related systems are given.
- You should be able to justify
the trends in this data !
Why are the protons adjacent to carbonyl
groups acidic ?
As we have advocated before, look at the stabilisation
of the conjugate base.
Notice the proximity of the adjacent p system, and hence the possibility for
RESONANCE stabilisation by delocalisation of the negative charge to the
more electronegative oxygen atom.
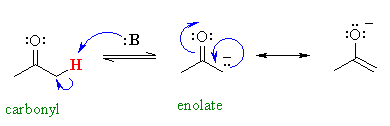
|
The more effective the resonance
stabilisation of the negative charge, the more stable the conjugate base
is and therefore the more acidic the parent system.
|
Let's compare pKa of the
common systems: aldehyde pKa = 17, ketone pKa = 19 and
an ester pKa = 25, and try to justify the trend.
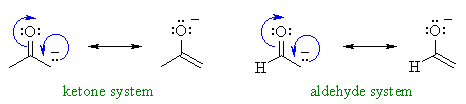
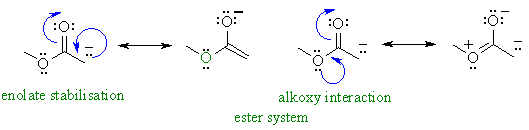 The difference between the 3 systems
is in the nature of the group attached to the common carbonyl. The aldehyde
has a hydrogen, the ketone an alkyl- group and the ester an alkoxy- group.
The difference between the 3 systems
is in the nature of the group attached to the common carbonyl. The aldehyde
has a hydrogen, the ketone an alkyl- group and the ester an alkoxy- group.
- H atoms are regarded as having
no electronic effect : they don't withdraw or donate electrons.
- Alkyl groups are weakly electron
donating, they tend to destabilise anions (you should recall
that they stabilise carbocations). This is because they will be "pushing"
electrons towards a negative system which is unfavourable electrostatically.
Hence, the anion of a ketone, where there are extra alkyl groups is less
stable than that of an aldehyde, and so, a ketone is less acidic.
- In the ester, there is also a
resonance donation from the alkoxy group towards the carbonyl that competes
with the stabilisation of the enolate charge. This makes the ester enolate
less stable than those of aldehydes and ketones so esters are even
less acidic.