| Chapter 22: Amines |
| Chapter 22: Amines |
Summary
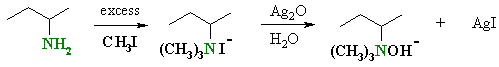
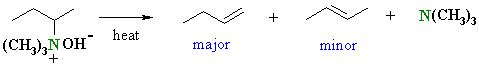
|
|
|
| The initial steps are an example
of the alkylation of an amine by methyl iodide.
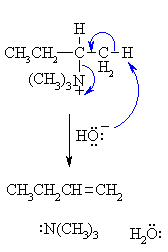
The mechanism of the elimination step is shown.
When heated, the hydroxide removes the more accessible proton, the π bond of the alkene C=C forms and the leaving group, a neutral amine departs. |
 |
| © Dr. Ian Hunt, Department of Chemistry |