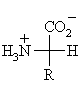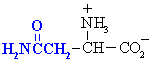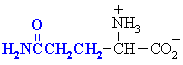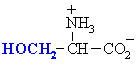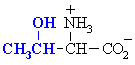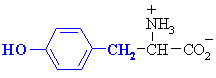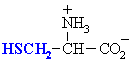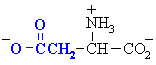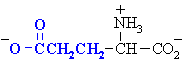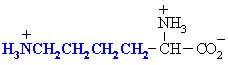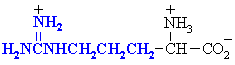 |
Chapter 27: Amino Acids, Peptides and Proteins |
 |
α-Amino Acids
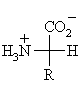
The generic structure of an α-amino acid
-
The 20 naturally occurring α-amino acids used
by cells to synthesise proteins can be generally represented by the generic
formula shown above.
-
The means the main difference between the various amino acids lies in the
structure of the "R" group.
-
These 20 α-amino acids can be sub-classified
according to how the properties of other functional groups in the "R"
group influence the system.
QUESTION Which common amino acid doesn't quite fit the generic
formula ? ANSWER
Amino acids with non-polar side chains. The
hydrophobic side chains are chemically unreactive and tend to aggregate
rather than be exposed to the aqueous environment, so they tend to found
on the interior of proteins. Hydrophobic means "water hating"
- remember "oil and water don't mix" and "like dissolves like" - this is
because non-polar hydrocarbons do not interact with polar water molecules
in an energetically favourable way - they would rather interact with another
non-polar hydrocarbon molecule : this it the hydrophobic effect
- the aggregation of non-polar systems in an aqueous environment.
(see also "micelles")
|
Amino acid
|
Abbreviations |
Structural Formula
|
3D-representation
|
|
Glycine
|
Gly (G)
|
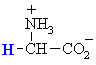
|
|
|
Alanine
|
Ala (A)
|
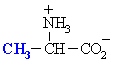
|
|
|
Valine
|
Val (V)
|
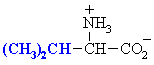
|
|
|
Leucine
|
Leu (L)
|
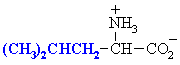
|
|
|
Isoleucine
|
Ile (I)
|
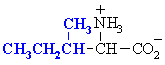
|
|
|
Methionine
|
Met (M)
|
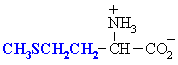
|
|
|
Proline
|
Pro (P)
|
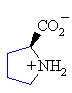
|
|
|
Phenylalanine
|
Phe (F)
|
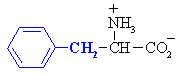
|
|
|
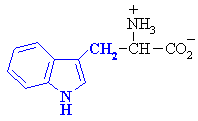
Tryptophan
|
Trp (W)
|
|
|
Amino acids with polar side chains.
These are side chains can be involved in hydrogen bonding interactions.
Cysteine is important because of its ability to form disulfide bonds.
|
Amino acid
|
Abbreviations |
Structural Formula
|
3D-representation
|
|
Asparagine
|
Asn (N)
|
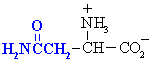
|
|
|
Glutamine
|
Gln (Q)
|
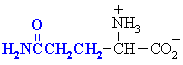
|
|
|
Serine
|
Ser (S)
|
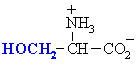
|
|
|
Threonine
|
Thr (T)
|
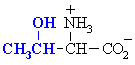
|
|
|
Tyrosine
|
Tyr (Y)
|
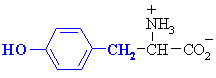
|
|
|
Cysteine
|
Cys (C)
|
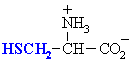
|
|
Amino acids with acidic side chains. These
carboxylate group will be -ve at physiological pH. Often involved
at the active sites of enzymes, in hydrogen bonding interactions and in
acid/base type reactivity.
|
Amino acid
|
Abbreviations |
Structural Formula
|
3D-representation
|
|
Aspartic acid
|
Asp (D)
|
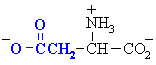
|
|
|
Glutamic acid
|
Glu (E)
|
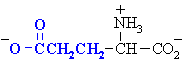
|
|
Amino acids with basic side chains. Often
involved at the active sites of enzymes, in hydrogen bonding interactions
and in acid/base type reactivity (e.g. histidine)
|
Amino acid
|
Abbreviations |
Structural Formula
|
3D-representation
|
|
Lysine
|
Lys (K)
|
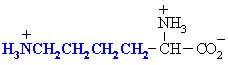
|
|
|
Arginine
|
Arg (R)
|
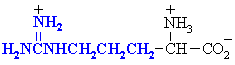 |
|
|
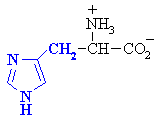
Histidine
|
His (H)
|
|
|