| Chapter 27: Amino Acids, Peptides, Proteins and Nucleic Acids |
| Chapter 27: Amino Acids, Peptides, Proteins and Nucleic Acids |
Structure and pKa of Amino Acids
|
|
|
||
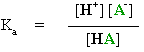 |
|
||
From these expressions it is possible to derive the important Henderson-Hasselbalch equation :
pKa = pH + log [HA] / [A-]
How does this equation help us ?

 These
principles can be extended to poly acidic / basic systems (such as amino
acids) by thinking of each pKa value in turn.
These
principles can be extended to poly acidic / basic systems (such as amino
acids) by thinking of each pKa value in turn.
This information will let you decide which structure of an acid
or base will dominate at a particular pH.
Let's look at an example.
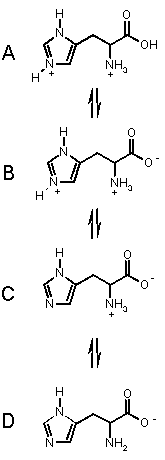
To the left are the processes for the amino acid HISTIDINE which has an extra basic group.
It has three acidic groups of pKa's 1.82 (carboxylic acid), 6.04 (pyrrole
NH) and 9.17 (ammonium NH).
Histidine can exist in the four forms shown, depending on the solution
pH, from acidic pH (top) to basic pH. (bottom).
Starting from the top, we can imagine that as we add base, the most acidic proton is removed first (COOH), then the pyrrole NH then finally the amino NH. These takes us through each of the forms in turn.
At pH < 1.82, A is the dominant form.
In the range 1.82 < pH < 6.02 B is the dominant form.
In the range 6.02 < pH < 9.17 C is the dominant form, and
when pH > 9.17, D is the major form in solution.
| © Dr. Ian Hunt, Department of Chemistry |