| Useful Concepts |
| Useful Concepts |
First, consider the general equation of a simple acid reaction:
The more stable the conjugate base, A-, is then the more the equilibrium favours
the product side.....
The more the equilibrium favours products, the more H+ there
is....
The more H+ there is then the stonger H-A is as an acid....
So looking for factors that stabilise the conjugate base, A-, gives us a way to deduce acidity.
For a discussion of the factors that influence acidity, see the page on acidity
Here is a link to an acidity ladder.... a diagram that schematically shows the approximate pKa values of important systems for organic chemistry.
Basicity
Consider the same basic equation as used for acidity, but think about the factors
that affect the availability of the electrons. Afterall, bases are electron
pair donors.
The factors are the same ones that affect acidity. For example a more electronegative
atom is a poorer electron donor and therefore a weaker base.
Structure and pKa
The information here is to help you decide which
structure of an acid or base will dominate at a particular pH. Let's do a general
case.
 The
equation for an acid is just HA = H+ + A- where
= means equilibrium
The
equation for an acid is just HA = H+ + A- where
= means equilibrium
pKa is defined as -log10 Ka where Ka = [H+][A-]
/ [HA].
From these expressions it is possible to derive the Henderson-Hasselbalch equation
which is
pKa = pH + log [HA] / [A-]
This tells us that when the pH = pKa then log [HA] / [A-]
= 0 therefore [HA] = [A-] ie equal amounts of the two forms.
If we make the solution more acidic, ie lower the pH, then pH < pKa and log [HA] / [A-] has to be > 0 so [HA] > [A-].
This makes sense as it tells us that the protonated form dominates in an acidic
medium.
If instead we make the solution more basic, ie raise the pH, then pH
> pKa and log [HA] / [A-] has to be < 0 so [HA]
<[A-]. This makes sense as it tells us that the deprotonated form
dominates in the basic medium.
These principles can be extended to poly acidic / basic systems (such as amino acids) by thinking of each pKa value in turn.
Lets look at an example.
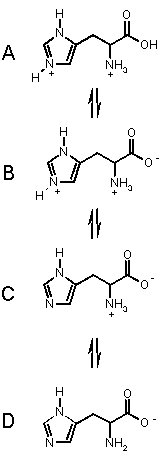
To the right are the processes for the amino acid
HISTIDINE, which has three acidic groups of pKa's 1.82 (carboxylic
acid) 6.04 (pyrrole NH) and 9.17 (ammonium NH). Histidine can exist in the four
forms shown, depending on the solution pH, from acidic pH (top) to basic pH.
(bottom).
Starting from the top, we can imagine that as we add base, the most acidic proton
is removed first (COOH), then the pyrrole NH then finally the amino NH. These
takes us through each of the forms in turn.
At pH < 1.82, A is the dominant form.
In the range 1.82 < pH < 6.02 B is the dominant form.
In the range 6.02 < pH < 9.17 C is the dominant form, and when pH > 9.17, D is the major form in solution. OK?
| © Dr. Ian Hunt, Department of Chemistry |