Part
5: STRUCTURE DETERMINATION
a. The empirical formula is the simplest ratio of the elements therefore C8H10 has an empirical formula = C4H5. (1 mark)
b. For a hydrocarbon, the formula for the index of hydrogen deficiency, IHD = 0.5 ( 2c+2-h), therefore, the IHD = 0.5 (2 x 8 + 2 - 10) = 4 (1 mark)
FYI : The IHD = pi + r (pi bonds plus rings)
there can be 4 units of unsaturation, which could mean combinations of rings, double and triple bonds.
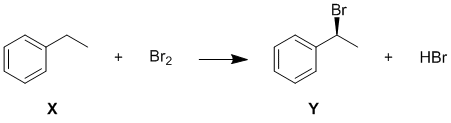
c. The IR data (1500 and 1600 cm-1) indicates likely aromatic C=C and (2900-3100 cm-1) sp2 and sp3 C-H. In order to give a single chiral monobromination product with only 5 H and 6 C types, X must be ethyl benzene, structure shown below. (2 marks for X)
d. The balanced equation is: (in addition to the marks for X in c, 2 marks for reagent, Y and the by-product). Note the question just asked for an equation NOT a mechanism.

Y is the major product because radical substitution which only occurs at sp3 C-H (note sp2 C-H bonds are stronger than sp3 C-H). When using bromine, the reaction will proceed via cleavage of the weakest C-H bond and hence the most stable radical. In this case, that means the secondary, benzylic radical is formed. This is a resonance stabilised radical. (1.5 marks)
e.The simplest 3D representation would be a simple wedge/hash diagram as shown below. There are of course other ways to draw it. The question only required that you draw one structure and name it. (3D structure = 1 mark, name = 1.5 marks)
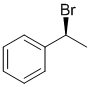 (S)-1-bromo-1-phenylethane or (S)-(1-bromoethyl)benzene
(S)-1-bromo-1-phenylethane or (S)-(1-bromoethyl)benzene
f. Z has the same molecular formula as X. The IR data tells us that Z has an alkyne C≡C (2120 cm-1) and that it is a terminal alkyne due to the sp C-H (3300 cm-1). Since Z has only 3 H and 4 C types, it must be symmetrical. (2 marks)
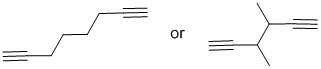
g.
Looking for a base that will deprotonate the terminal alkyne means we need a base with pKa > or = 25. Good choices would be amide ion (-NH2), or hydride (H-). (1 mark)
Common errors:
Not reading the questions.... so drawing structures that did not fit the molecular formula, not writing a balanced equation (attempting to drawing a mechanism instead),
Did not know what an empirical formula was the simplest ratio of the elements. (High school / 1st yr knowledge)
Incorrectly counting or not checking the number of H and or C types in Y and / or Z.
Drawing structures for X that did not fit the molecular formula of C8H10 and the IHD.
Note that IHD defines the number of rings + pi bonds present for a given molecular formulae. The answer to part b should have helped you avoid this!
Could not name Y correctly, ignoring the chirality that was specifically referred to. Hence but be named as R or S!
Incorrect numbering, incorrect alphabetisation, wrong root name (e.g. wrong root, mixing up phenyl with benzyl).
Did not write a balanced equation including HBr as the by product. Not recognising it as a radical substitution.
Drawing structures for Z that did not fit all the data provided (esp. molecular formula, IR 3300 cm-1 and number of H and C types)
Suggesting the conjugate acid rather than the base or bases that are not strong enough to deprotonate terminal alkynes (such as HO- or RO-). Think about what the pKa refers to. Amines (e.g. NH3 or RNH2) have pKa about 10.