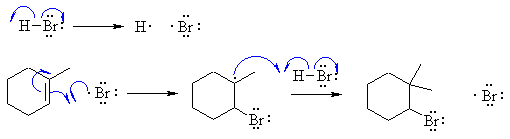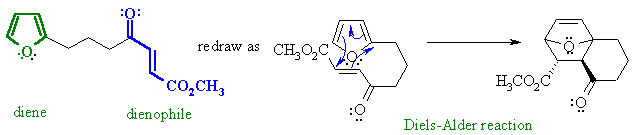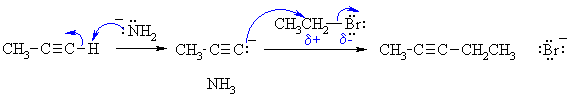
Notes :
1. NaNH2 is essentially ionic in that it can be thought of as Na+ and -NH2 . If you consider it as ionic with formal full charges, don't show a covalent bond.
2. Since the alkyl halide here is primary, it will NOT form a carbocation so the reaction is not an SN1.
3. Remember, electron rich to electron poor.
1. NaNH2 is essentially ionic in that it can be thought of as Na+ and -NH2 . If you consider it as ionic with formal full charges, don't show a covalent bond.
2. Since the alkyl halide here is primary, it will NOT form a carbocation so the reaction is not an SN1.
3. Remember, electron rich to electron poor.