| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
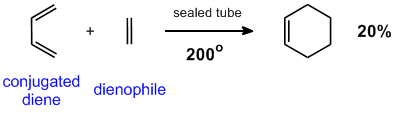
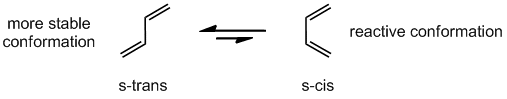
Diels-Alder Reaction (discovered 1928, Nobel Prize in 1950)


| Dienes | 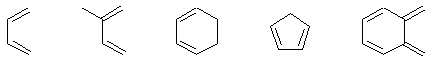 |
| Dienophiles | 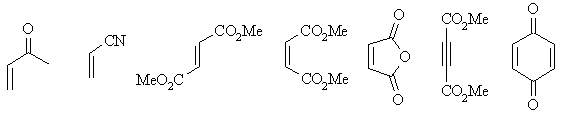 |
Study Tips:
|
Stereoselectivity:
a cis-dienophile gives cis-substituents
in the product |
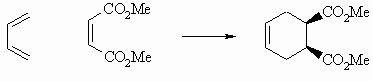 |
||
a trans-dienophile gives trans-substituents
in the product. |
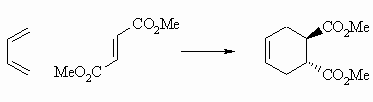 |
||
If the diene substituents have the same stereochemistry (here they are both
E), then both diene substituents end up on the same face of the product. |
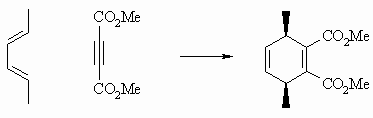 |
||
If the diene substituents have opposite stereochemistry (here one is E and one Z), then the diene substituents end up on opposite faces of the product. |
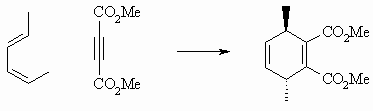 |
Cyclic dienes can give stereoisomeric products depending on whether the dienophile lies under or away from the diene in the transition state. The endo product is usually the major product (due to kinetic control)
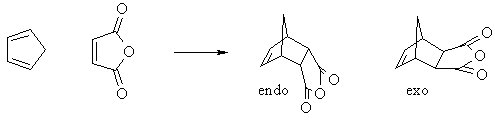 |
||
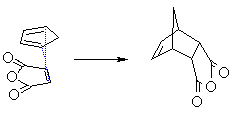 Diene and dienophile aligned directly over each other gives the endo product (dienophile under or in = endo) |
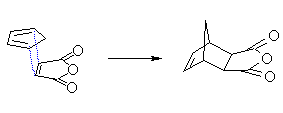 Diene and dienophile staggered with respect to each other gives the exo product (dienophile exposed or out = exo) |
|
|
|
|
|
Compare the relative position of the dienophile fragment
in the JSMOL images above. |
||
| © Dr. Ian Hunt, Department of Chemistry |