 |
Chapter 1: Structure Determines
Properties |
 |
Orbitals Quantum numbers
The properties of orbitals (energy, size, shape etc.) and the
electrons within the orbitals (location, spin) are described using
quantum
numbers:
| quantum number |
symbol |
value range |
describes
|
|
principal
|
n
|
1, 2, 3, .... |
energy level |
| angular momentum |
l
|
0 to n-1 |
orbital shape (s = 0, p = 1, d = 2 etc.) |
|
magnetic
|
ml
|
- l to + l |
spatial orientation and degeneracy
(number of orbitals of that type) |
|
spin
|
ms
|
± 1/2 |
electron spin |
Given an orbital, the quantum
numbers that describe that orbital can
be determined, or, given a set of quantum numbers one can also
determine
the type of orbital :
|
n
|
l
|
ml
|
orbital |
|
1
|
0
|
0
|
1s
|
|
2
|
0
|
0
|
2s
|
|
1
|
-1
|
2px
|
|
0
|
2py
|
|
+1
|
2pz
|
Electron Configurations
e.g. Consider the
electron configuration of Al, which is the
13th atom in the periodic table, so
atomic
number Z = 13 and therefore is has 13 electrons:
A simple electron
configuration lists all the orbitals
and their electron occupancy
1s22s22p63s23p1
A short form of the above collapses the appropriate noble gas
configuration into the atomic symbol for the noble gas
[Ne]3s23p1
Orbital energy diagrams are usually written with a collapsed
core. They are used to indicate how exactly each individual
valence
orbital is being filled with electrons.
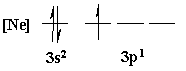
Orbital
Filling
Electron configurations can be
determined using a periodic table and
by considering the following as a guide to how the electrons "fill-up"
the orbitals:
Aufbau (Building-Up) Principle.
Electrons are placed into the atomic orbitals from lowest to highest energy.
The order of these orbitals is determined using the Schrodinger equation and
can be easily determined by looking at the periodic
table or using a simple mnemonic.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p etc......
Hund's rule of Maximum Multiplicity. When degenerate orbitals are
being filled, single electrons are placed into each degenerate orbital before
they are paired with another electron in the same orbital.
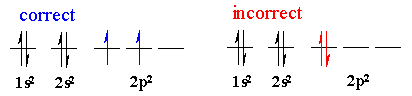
Pauli Exclusion Principle. No two electrons in a atom can have
the same four quantum numbers . This means that only two electrons are allowed
in the same orbital, and then they have opposite spin, +1/2 and -1/2.
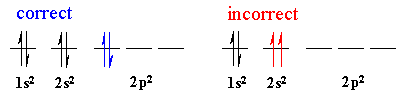
Important distinction:
Valence Electrons are the electrons
which are involved in bonding between atoms. They possess the highest
energy and are referred to as the 'outermost' electrons (keep in mind that does
not mean they cannot travel close to the nucleus at anyone time but they will
be the electrons which travel furthest away from the nucleus).
Core electrons are electrons that under 'normal'
reaction conditions
are chemically inert. They are the electrons of an atom which are
located in the completely filled energy levels.
 |
© Dr. Ian Hunt,
Department of Chemistry, University of Calgary |
 |

