| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
In order to view and use the interactive 3D structures on this site, you will need "JAVASCRIPT" enabled JSMOL can run. A simple automatic check was run so if you didn't get an error message, it should be good!
These images can be rotated and resized, you can also measure distances and angles etc. as well as changing the display mode. Some are animations of chemical processes. Check here if you want more details on using JSMOL.
When determining the shape of a molecule the premise is that groups of electrons surrounding an atom are repelled as far as possible from each other to minimize the overall energy of the molecule. Groups of electrons can refer to bonding or non-bonding valence electrons. The number and types of groups are determined from a Lewis diagram.
Not only is it important for a chemist to be able to visualize a structure, they must also be able to effectively (clearly!) transfer this knowledge onto a piece of paper!!!!!!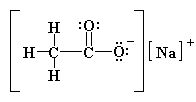
A molecule is visualized as if it were centered in the plane of the paper and, generally, so that the most number of atoms possible are in that plane. Unbroken lines are used to represent bonds in the plane of the paper, hashed wedges represent bonds going back out of the plane of paper and solid wedges represent bonds coming out of the plane of the paper.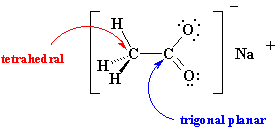
There may be several different ways that you can represent a molecule on a piece of paper. You should be able to discriminate which of those drawings most clearly represent the structure of a molecule.
You should be able to determine the
shape of a molecule by looking at its molecular formula.
In doing so you must be able to present clear drawings of your
conclusions.
If you think you can do this already, then try some questions.
Otherwise you will need to review VSEPR and maybe
how to draw molecules showing the 3D.
Molecular dipole moments
| Once the 3-dimensional
shape of a molecule is known, the molecular dipole can be
determined by adding together the vectors of the individual bond
dipoles. Note that just because a molecule has polar bonds, it doesn't mean there is a molecular dipole. For example molecules like carbon dioxide and carbon tetrachloride have polar bonds but no net molecular dipole. |
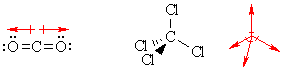 |
You should be able to indicate a molecule's polarity.
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |