| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
Resonance
In certain cases, molecules can be represent by more than
one reasonable Lewis structure that differ only in the location of π electrons.
Electrons in σ bonds have a fixed location and so they are said to be localised.
In contrast, π electrons that can be drawn in different locations are
said to be delocalised.
Collectively these Lewis diagrams are then known as resonance structures
or resonance contributors or resonance canonicals.
The "real" structure has characteristics of each of the contributors, and is
often represented as the resonance hybrid (think of a hybrid breed which
is a mixed breed). In a way, the resonance hybrid is a mixture of the
contributors.
(note that a resonance hybrid cannot normally be written as an individual Lewis diagram !).
You should be able to draw all reasonable resonance structures for a given organic molecule. Examples.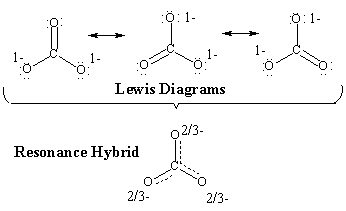
The best way to "derive" resonance structures is by learning
to "push" curly arrows and starting from a reasonable
Lewis structure.
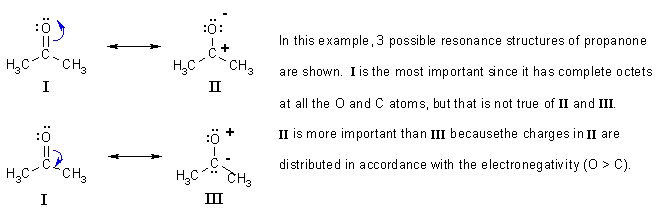
Here is an organic example. Note how the possible resonance structures are derived from the starting point by pushing curly arrows.
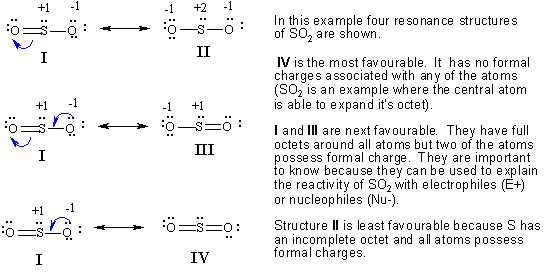
Now would be a good time to check out some questions
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |