| Chapter 3: Conformations of Alkanes and Cycloalkanes |
| Chapter 3: Conformations of Alkanes and Cycloalkanes |
Energetics
The predominant forces involved in chemistry are electrical in origin based on the physics associated with Coulomb's Law of electrostatics. The basics are reviewed below:The force between two charged particles q1 and
q2 is inversely proportional to the distance, r, between them.
As an example, the attraction of electrons to an atomic nucleus. As an example, electron pair repulsion used in VSEPR |
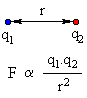 |
IMPORTANT IMPLICATIONS : Electron pairs repel each other. As a result, molecules are most stable when pairs of electrons are as far apart from each other as possible. When the pairs of electrons are too close together, then the molecule is destabilised and it is at higher energy. Remember, the most stable states are those of lowest energy.
(You should already know this from General Chemistry and Chapter 1, VSEPR.)
Conformational analysis is essentially an investigation
of forces and energies associated with the interactions of pairs of electrons
(these could be pairs of electrons in bonds or lone pairs). Strain is the term used to for the energy associated
with a system due to its geometry.
There are various types of strain that we need to be familar with. These and
associated terms are described below:
| © Dr. Ian Hunt, Department of Chemistry |