|
|
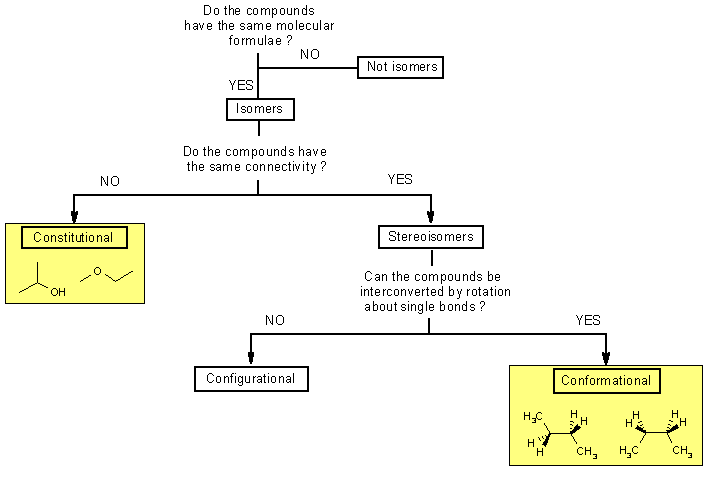
Isomers are compounds
with the same molecular formulae but that are structurally different
in some way. It is important to be able to recognise isomers because
they can have different chemical, physical properties and biological
properties.
|
|
Constitutional
isomers differ in the order in which the atoms are connected so they
can contain different functional groups and / or bonding patterns (e.g.
branching)
- example: 1-propanol, 2-propanol and ethyl
methyl ether (C3H8O)
|
|
Stereoisomers
have the same functional groups and connectivities, they differ only
in the arrangement of atoms and bonds in space.
|
|
Conformational
isomers (or conformers or rotational isomers or rotamers)
are stereoisomers produced by rotation about σ bonds, and are often
rapidly interconverting at room temperature
- example 1: butane : anti (left)
and syn (center). The C2-C3 σ bond rotation is animated (right).
Try rotating the model to look along the C-C to see the two forms.
- example 2: cyclohexane : chair (left)
and boat (right).These two forms can be interconverted by twisting
the ring structure.
|