| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
Radical Substitution Mechanism
Unlike the large majority of reactions that you will see in your organic chemistry course, radical mechanisms require that fishhook curly arrows that represent the motion of a single electron are used. These can be a little more confusing and more difficult to master. We suggest you get to grips with normal curved arrows first.
However, the mechanism for the bromination of methane is shown below, but the mechanism for chlorination or higher alkanes in the same. Note that it contains three distinct type of steps, depending on the net change in the number of radicals that are present.
| RADICAL CHAIN MECHANISM FOR REACTION
OF METHANE WITH Br2 |
|
| Step 1 (Initiation) Heat or uv light cause the weak halogen bond to undergo homolytic cleavage to generate two bromine radicals and starting the chain process. |
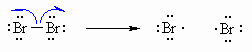 |
| Step 2 (Propagation) (a) A bromine radical abstracts a hydrogen to form HBr and a methyl radical, then (b) The methyl radical abstracts a bromine atom from another molecule of Br2 to form the methyl bromide product and another bromine radical, which can then itself undergo reaction 2(a) creating a cycle that can repeat. |
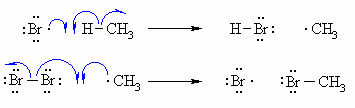 |
| Step 3 (Termination) Various reactions between the possible pairs of radicals allow for the formation of ethane, Br2 or the product, methyl bromide. These reactions remove radicals and do not perpetuate the cycle. |
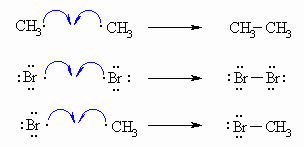 |
| © Dr. Ian Hunt, Department of Chemistry |