| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
Radical Halogenation of Alkanes
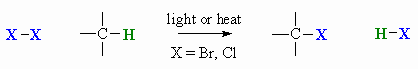
Summary:
| RADICAL CHAIN MECHANISM FOR REACTION
OF METHANE WITH Br2 |
|
| Step 1 (Initiation) Heat or uv light cause the weak halogen bond to undergo homolytic cleavage to generate two bromine radicals and starting the chain process. |
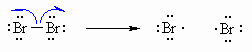 |
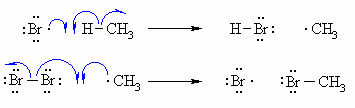
| Step 2 (Propagation) (a) A bromine radical abstracts a hydrogen to form HBr and a methyl radical, then (b) The methyl radical abstracts a bromine atom from another molecule of Br2 to form the methyl bromide product and another bromine radical, which can then itself undergo reaction 2(a) creating a cycle that can repeat. |
 |
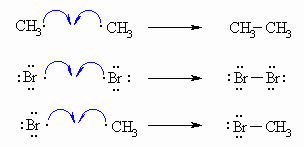
| Step 3 (Termination) Various reactions between the possible pairs of radicals allow for the formation of ethane, Br2 or the product, methyl bromide. These reactions remove radicals and do not perpetuate the cycle. |
 |
More highly brominated by-products are possible if methyl bromide reacts with a bromine radical in the same fashion as methane does. Can you draw the cycle that leads to the formation of dibromomethane ?
| © Dr. Ian Hunt, Department of Chemistry |