| Chapter 6: Reactions of Alkenes: Addition Reactions |
| Chapter 6: Reactions of Alkenes: Addition Reactions |
Markovnikov's Rule
This is an empirical rule based on Markovnikov's experimental observations on the addition of hydrogen halides to alkenes. The rule states that :
This is illustrated by the following example: 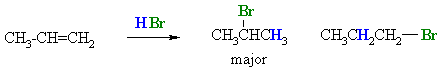
Look at the position of the H and the Br in relation to the statement of Markovnikovs rule given above. Note that the major product, which is often referred to as the Markovnikov product, is the more highly substituted alkyl halide. In the example above, the secondary bromide is formed in preference to the primary bromide.
|
|
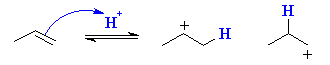
Here we see that in principle, propene can protonate to give two different carbocations, one 2o and the other 1o. |
|
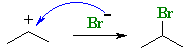
|
Although Markovnikov's rule was developed for and is specifically applied to the addition of hydrogen halides to alkenes, many other additions are also described as Markovnikov or anti-Markovnikov depending on the regioselectivity of the addition reaction, e.g. the acid catalysed hydration of alkenes (Markovnikov) and the hydroboration / oxidation of alkenes (anti-Markovnikov).
In more general terms, Markovnikov's rule can be "modernised" to cover other addition reactions by considering that the electrophile adds to the least substituted end of the alkene giving rise to the more stable intermediate. So let's rephrase our statement of Markovnikov's rule:
"when an unsymmetrical alkene undergoes addition with E-Nu, then the electrophile, E, adds to the carbon of the alkene that has the greater number of hydrogen substituents, and the nucleophile, Nu, to the carbon of the alkene with the fewer number of hydrogen substituents"
| Therefore, the key is to recognise the electrophilic portion of the reagent as it adds to the π bond first so as to give the more stable intermediate. |
| © Dr. Ian Hunt, Department of Chemistry |