| Chapter 6: Reactions of Alkenes: Addition Reactions |
| Chapter 6: Reactions of Alkenes: Addition Reactions |
Hydroboration / Oxidation of Alkenes
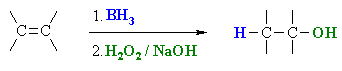
Summary
 |
The image shows the electrostatic potential map for borane,
BH3. The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density.
|
 |
|
Related reactions
| MECHANISM FOR REACTION OF ALKENES WITH BH3 | |
| Step 1: A concerted reaction. The π electrons act as the nucleophile with the electrophilic B and the H is transferred to the C with syn stereochemistry. |
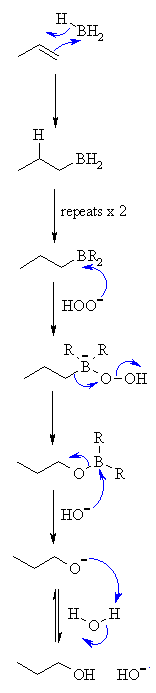 |
| Step 2: First step repeats twice more so that all of the B-H bonds react with C=C |
|
| Step 3: Peroxide ion reacts as the nucleophile with the electrophilic B atom. |
|
| Step 4: Migration of C-B bond to form a C-O bond and displace hydroxide. Stereochemistry of C center is retained. |
|
| Step 5: Attack of hydroxide as a nucleophile with the electrophilic B displacing the alkoxide. |
|
| Step 6: An acid / base reaction to form the alcohol. |
|
| © Dr. Ian Hunt, Department of Chemistry |