| Chapter 6: Reactions of Alkenes: Addition Reactions |
| Chapter 6: Reactions of Alkenes: Addition Reactions |
Halohydration of Alkenes

Summary
| MECHANISM FOR REACTION OF ALKENES WITH Br2 / H2O | |
|
Step 1: |
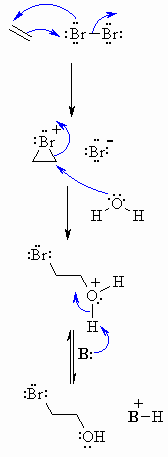 |
| Step 2: Attack of the nucleophilic water molecule from the side away from the bromonium center opens the cyclic bromonium ion to give overall trans addition. |
|
| Step 3: An acid / base reaction converts the oxonium into the alcohol. |
|
| © Dr. Ian Hunt, Department of Chemistry |