| Chapter 7 : Stereochemistry |
| Chapter 7 : Stereochemistry |
The Cahn-Ingold-Prelog R/S rules are used for naming enantiomers and diastereomers
NB: The term chirality has superceded the term stereogenic or chiral center.
More about the position of the observer... (i.e. the requirement that you need to put the low priority group away from you)
Example: bromofluoroiodomethane
| The chirality center is easy to spot, and the four attached groups are I (purple), Br (maroon), F(green) and H (white), listed in priority order, highest to to lowest. Rotate the JSMOL image on the left so the you are looking along the C-H bond and the H is away from you, then determine the direction of high to low priority. It decreases clockwise (I to Br to F), so this is the R enantiomer. |
|
|
|
Subrules:
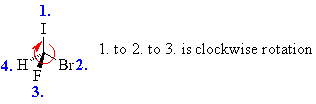
| © Dr. Ian Hunt, Department of Chemistry |